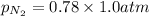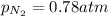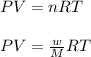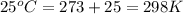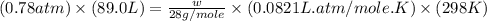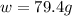Chemistry, 10.03.2020 01:16 andrew2786
Calculate the mass of nitrogen dissolved at room temperature in an 89.0 LL home aquarium. Assume a total pressure of 1.0 atmatm and a mole fraction for nitrogen of 0.78.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 19:30
⁉️how many kj of energy would be needed to convert 150. g of ammonia to vapor at its boiling point? ⁉️(ammonia’s heat of vaporization is 1.38 kj/g
Answers: 3

Chemistry, 23.06.2019 23:00
For a particular first-order reaction, it takes 24 minutes for the concentration of the reactant to decrease to 25% of its initial value. what is the value for rate constant (in s-1) for the reaction?
Answers: 2
You know the right answer?
Calculate the mass of nitrogen dissolved at room temperature in an 89.0 LL home aquarium. Assume a t...
Questions



Law, 16.03.2022 14:00




SAT, 16.03.2022 14:00

History, 16.03.2022 14:00

Mathematics, 16.03.2022 14:00

Geography, 16.03.2022 14:00










Mathematics, 16.03.2022 14:00

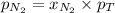
 = partial vapor pressure of nitrogen = ?
= partial vapor pressure of nitrogen = ?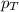 = total pressure = 1.0 atm
= total pressure = 1.0 atm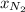 = mole fraction of nitrogen = 0.78
= mole fraction of nitrogen = 0.78