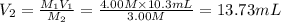Chemistry, 10.03.2020 01:07 jocelyngracia
A chemist must dilute 10.3mL of 4.00M aqueous sodium chloride NaCl solution until the concentration falls to 3.00M . He'll do this by adding distilled water to the solution until it reaches a certain final volume.
Required:
Calculate this final volume, in liters.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
A chemist must dilute 10.3mL of 4.00M aqueous sodium chloride NaCl solution until the concentration...
Questions




History, 24.04.2020 07:19

History, 24.04.2020 07:19

Mathematics, 24.04.2020 07:19

History, 24.04.2020 07:20


Physics, 24.04.2020 07:20





History, 24.04.2020 07:20

Social Studies, 24.04.2020 07:20

English, 24.04.2020 07:20


Mathematics, 24.04.2020 07:20

Mathematics, 24.04.2020 07:20

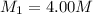
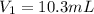
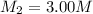
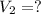
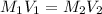 (dilution)
(dilution)