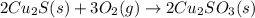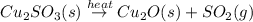Chemistry, 10.03.2020 00:40 leysirivera23ovez6n
When copper sulfide is partially roasted in air (reaction with o2), copper sulfite is formed first. subsequently, upon heating, the copper sulfite thermally decomposes to copper oxide and sulfur dioxide. Write balanced chemical equations for these two reactions

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
When copper sulfide is partially roasted in air (reaction with o2), copper sulfite is formed first....
Questions


Geography, 04.05.2021 03:10

Biology, 04.05.2021 03:10

English, 04.05.2021 03:10

Chemistry, 04.05.2021 03:10

Mathematics, 04.05.2021 03:10

Spanish, 04.05.2021 03:10









Mathematics, 04.05.2021 03:10

History, 04.05.2021 03:10

Computers and Technology, 04.05.2021 03:10

Spanish, 04.05.2021 03:10