Chemistry, 10.03.2020 00:37 serenityarts123
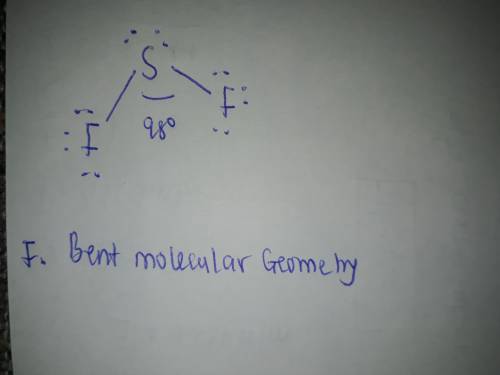
Draw the Lewis structure of SF2, showing all lone pairs. Identify the molecular geometry of SF2.
A. square pyramidal
B. trigonal pyramidal
C. trigonal planar
D. linear
E. T‑shaped
F. bent
G. square planar
H. octahedral
I. trigonal bipyramidal
J. tetrahedral

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
Draw the Lewis structure of SF2, showing all lone pairs. Identify the molecular geometry of SF2.
Questions







Mathematics, 13.04.2021 18:30


Mathematics, 13.04.2021 18:30


History, 13.04.2021 18:30

Mathematics, 13.04.2021 18:30

English, 13.04.2021 18:30


English, 13.04.2021 18:30

Chemistry, 13.04.2021 18:30



Mathematics, 13.04.2021 18:30