
Chemistry, 10.03.2020 00:50 rebeccacruzz2017
Assume that for the pH 9.2 phenolphthalein sample, you used half as much phenolphthalein solution (i. e your sample is 9 ml buffer, 0.5 ml phenophthalein solution and 0.5 ml water). what should happen to the value of Kc you determine?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
Assume that for the pH 9.2 phenolphthalein sample, you used half as much phenolphthalein solution (i...
Questions

Mathematics, 07.02.2022 06:00

SAT, 07.02.2022 06:00







Mathematics, 07.02.2022 06:00


World Languages, 07.02.2022 06:00


Mathematics, 07.02.2022 06:00

History, 07.02.2022 06:00


Mathematics, 07.02.2022 06:00

Mathematics, 07.02.2022 06:00

English, 07.02.2022 06:10


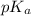 as follows.
as follows.![pK_{a} + log \frac{[H_{3}O^{+}]}{[\text{solution}]}](/tpl/images/0539/4001/906ab.png)
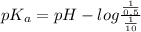
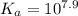
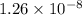
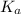 comes out to be positive. This means that there will occur an increase in the value of
comes out to be positive. This means that there will occur an increase in the value of 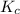 .
.

