
Chemistry, 10.03.2020 00:21 mcsaggers2842
Consider the reaction
5Br-(aq)+BrO-3(aq)+6H+(aq) ?3Br2(aq)+3H2O(l)
The average rate of consumption of Br- is 1.56 x10^-4M/s over the first two minutes.
a. What is the average rate of formation of Br2 during the same time interval?
b. What is the average rate of consumption of H+ during the same time interval?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
Consider the reaction
5Br-(aq)+BrO-3(aq)+6H+(aq) ?3Br2(aq)+3H2O(l)
The avera...
5Br-(aq)+BrO-3(aq)+6H+(aq) ?3Br2(aq)+3H2O(l)
The avera...
Questions




Mathematics, 23.06.2019 08:30






Mathematics, 23.06.2019 08:30





History, 23.06.2019 08:30


History, 23.06.2019 08:30

History, 23.06.2019 08:30

Biology, 23.06.2019 08:30

Mathematics, 23.06.2019 08:30

![\frac{d[Br_2]}{dt}=9.36\times 10^{-5}M/s](/tpl/images/0539/2538/cfec7.png)
![\frac{d[H^+]}{dt}=1.87\times 10^{-4}M/s](/tpl/images/0539/2538/14675.png)
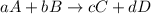
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0539/2538/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0539/2538/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0539/2538/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0539/2538/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0539/2538/d4b94.png)
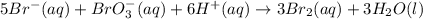
![\text{Rate of disappearance of }Br^-=-\frac{1}{5}\frac{d[Br^-]}{dt}](/tpl/images/0539/2538/86e92.png)
![\text{Rate of disappearance of }BrO_3^-=-\frac{d[BrO_3^-]}{dt}](/tpl/images/0539/2538/4a0f1.png)
![\text{Rate of disappearance of }H^+=-\frac{1}{6}\frac{d[H^+]}{dt}](/tpl/images/0539/2538/09d2b.png)
![\text{Rate of formation of }Br_2=+\frac{1}{3}\frac{d[Br_2]}{dt}](/tpl/images/0539/2538/81dff.png)
![\text{Rate of formation of }H_2O=+\frac{1}{3}\frac{d[H_2O]}{dt}](/tpl/images/0539/2538/a608b.png)
![\text{Rate of reaction}=-\frac{1}{5}\frac{d[Br^-]}{dt}=-\frac{d[BrO_3^-]}{dt}=-\frac{1}{6}\frac{d[H^+]}{dt}=+\frac{1}{3}\frac{d[Br_2]}{dt}=+\frac{1}{3}\frac{d[H_2O]}{dt}](/tpl/images/0539/2538/32ca3.png)
![\frac{1}{5}\frac{d[Br^-]}{dt}=1.56\times 10^{-4}M/s](/tpl/images/0539/2538/d9606.png)
![-\frac{1}{5}\frac{d[Br^-]}{dt}=+\frac{1}{3}\frac{d[Br_2]}{dt}](/tpl/images/0539/2538/7f11c.png)
![\frac{d[Br_2]}{dt}=\frac{3}{5}\frac{d[Br^-]}{dt}](/tpl/images/0539/2538/5d184.png)
![\frac{d[Br_2]}{dt}=\frac{3}{5}\times 1.56\times 10^{-4}M/s](/tpl/images/0539/2538/63026.png)
![-\frac{1}{5}\frac{d[Br^-]}{dt}=-\frac{1}{6}\frac{d[H^+]}{dt}](/tpl/images/0539/2538/b95e1.png)
![-\frac{1}{6}\frac{d[H^+]}{dt}=\frac{3}{5}\frac{d[Br^-]}{dt}](/tpl/images/0539/2538/fffef.png)
![\frac{d[H^+]}{dt}=\frac{6}{5}\times 1.56\times 10^{-4}M/s](/tpl/images/0539/2538/f226c.png)



