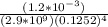Chemistry, 10.03.2020 00:22 toshahoskins0098
A solution is made that is 1.2×10−3 M in Zn(NO3)2 and 0.130 M in NH3. After the solution reaches equilibrium, what concentration of Zn2+(aq) remains?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
A solution is made that is 1.2×10−3 M in Zn(NO3)2 and 0.130 M in NH3. After the solution reaches equ...
Questions


Business, 06.05.2020 04:46

English, 06.05.2020 04:46









Mathematics, 06.05.2020 04:46

Biology, 06.05.2020 04:46

Social Studies, 06.05.2020 04:46


Mathematics, 06.05.2020 04:46




Biology, 06.05.2020 04:46

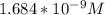
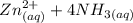 ⇄
⇄ ![[Zn(NH_3)_4^+_{(aq)}]](/tpl/images/0539/2581/6fbcb.png)
![[Zn^{2+}]](/tpl/images/0539/2581/9c01a.png) at equilibrium.
at equilibrium.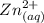

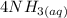 ⇄
⇄ 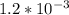
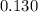
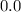

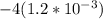

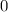
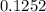
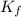

![\frac{[Zn(NH_3)_4^{2+}]}{[Zn^{2+}][NH_3]^4}](/tpl/images/0539/2581/585d3.png) ⇒
⇒ ![\frac{[Zn(NH_3)_4^{2+}]}{[K_fNH_3]^4}](/tpl/images/0539/2581/6256e.png)