Chemistry, 10.03.2020 01:00 jasbutt015p2pqp8
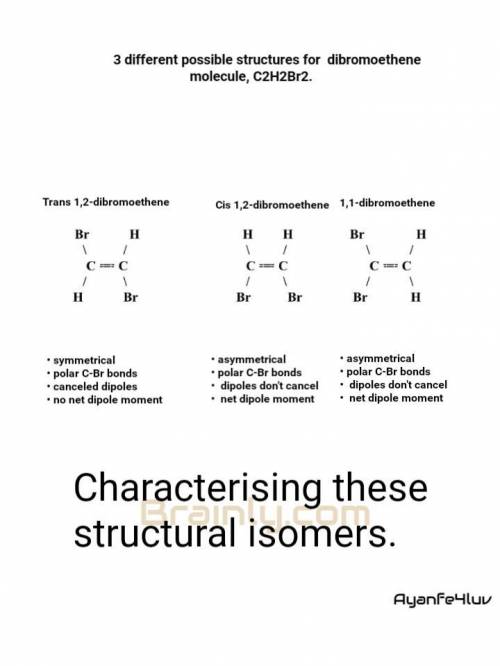
There are three different possible structures (known as isomers) of a dibromoethene molecule, C 2 H 2 Br 2 . One of them has no net dipole moment, but the other two do. Draw Lewis structures for each of these structures. Include H atoms.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 23.06.2019 09:00
The concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point
Answers: 3
You know the right answer?
There are three different possible structures (known as isomers) of a dibromoethene molecule, C 2 H...
Questions












English, 30.10.2020 17:00


Mathematics, 30.10.2020 17:00

History, 30.10.2020 17:00



Mathematics, 30.10.2020 17:00