
Chemistry, 09.03.2020 23:58 denaeyafranklin8430
Calculate the enthalpy of formation (kJ/mol) of MgO(s). The enthalpy of reaction for the equation as written is 100.8 kJ/mol. If the answer is negative, enter the sign and then the magnitude.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

You know the right answer?
Calculate the enthalpy of formation (kJ/mol) of MgO(s). The enthalpy of reaction for the equation as...
Questions



Mathematics, 20.11.2020 09:50




Chemistry, 20.11.2020 09:50





Biology, 20.11.2020 09:50


Mathematics, 20.11.2020 09:50


History, 20.11.2020 09:50


Social Studies, 20.11.2020 09:50

Mathematics, 20.11.2020 09:50

Mathematics, 20.11.2020 09:50

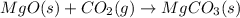
![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0539/1394/e893d.png)
![\Delta H_{rxn}=[(1\times \Delta H_f_{(MgCO_3(s))})]-[(1\times \Delta H_f_{(MgO(s))})+(1\times \Delta H_f_{(CO_2(g))})]](/tpl/images/0539/1394/01988.png)
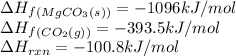
![-100.8=[(1\times (-1096))]-[(1\times \Delta H_f_{(MgO(s))})+(1\times (-393.5))]\\\\\Delta H_f_{(MgO(s))}=-601.7kJ/mol](/tpl/images/0539/1394/d8103.png)


