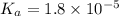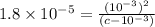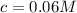Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

You know the right answer?
A typical sample of vinegar has a pH of 3.0. Assuming that vinegar is only an aqueous solution of ac...
Questions

Mathematics, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01


Mathematics, 16.08.2020 01:01


Biology, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01

Social Studies, 16.08.2020 01:01

History, 16.08.2020 01:01


Computers and Technology, 16.08.2020 01:01

Health, 16.08.2020 01:01

Chemistry, 16.08.2020 01:01


Mathematics, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01


Mathematics, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01

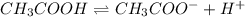
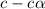
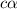
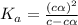
![pH=-log[H^+]](/tpl/images/0539/0805/15713.png)
![3.0=-log[H^+]](/tpl/images/0539/0805/d1dae.png)
![[H^+]=c\times \alpha=10^{-3}](/tpl/images/0539/0805/9452b.png)