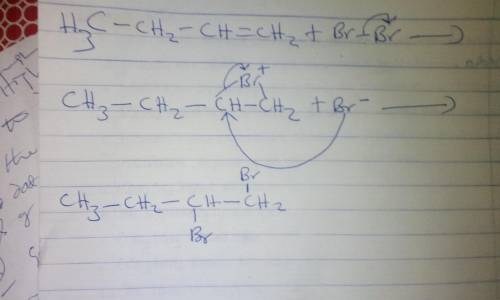
Electrophilic addition of bromine, Br2, to alkenes yields a 1,2-dibromoalkane. The reaction proceeds through a cyclic intermediate known as a bromonium ion. The reaction occurs in an anhydrous solvent such as CH2Cl2. In the second step of the reaction, bromide is the nucleophile and attacks at one of the carbons of the bromonium ion to yield the product. Due to steric clashes, the bromide ion always attacks the carbon from the opposite face of the bromonium ion so that a product with anti stereochemistry is formed. Draw curved arrows to show the movement of electrons in this step of the mechanism.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1

Chemistry, 23.06.2019 15:20
How many stereoisomers will be formed from the addition of phenyllithium to this molecule?
Answers: 1
You know the right answer?
Electrophilic addition of bromine, Br2, to alkenes yields a 1,2-dibromoalkane. The reaction proceeds...
Questions

Mathematics, 21.08.2019 08:00

Social Studies, 21.08.2019 08:00


Mathematics, 21.08.2019 08:00


Mathematics, 21.08.2019 08:00

Mathematics, 21.08.2019 08:00


Mathematics, 21.08.2019 08:00


Mathematics, 21.08.2019 08:00


English, 21.08.2019 08:00


Mathematics, 21.08.2019 08:00

Biology, 21.08.2019 08:00


Mathematics, 21.08.2019 08:00

Social Studies, 21.08.2019 08:00

Mathematics, 21.08.2019 08:00