Chemistry, 09.03.2020 20:19 florochoa217
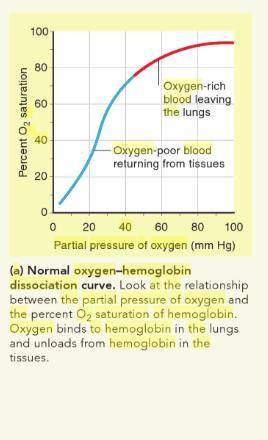
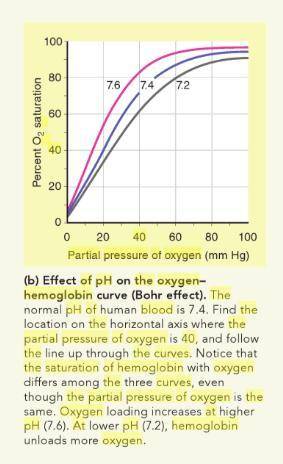
At normal blood pH pH (7.4), hemoglobin is 80 80 % saturated at a partial pressure of oxygen ( O 2 O2 ) of 40 mmHg 40 mmHg . Use the oxygen–hemoglobin dissociation curves to determine the oxygen saturation of hemoglobin at a partial pressure of O 2 O2 of 40 mmHg 40 mmHg , if the blood pH pH drops to 7.2.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
At normal blood pH pH (7.4), hemoglobin is 80 80 % saturated at a partial pressure of oxygen ( O 2 O...
Questions

History, 28.01.2020 01:31

English, 28.01.2020 01:31




Mathematics, 28.01.2020 01:31

Mathematics, 28.01.2020 01:31

History, 28.01.2020 01:31


Mathematics, 28.01.2020 01:31



Mathematics, 28.01.2020 01:31

Physics, 28.01.2020 01:31

History, 28.01.2020 01:31

History, 28.01.2020 01:31


Mathematics, 28.01.2020 01:31