Chemistry, 09.03.2020 18:50 lattimore12
Biphenyl, C12H10, is a nonvolatile, nonionizing solute that is soluble in benzene, C6H6. At 25 ∘C, the vapor pressure of pure benzene is 100.84 Torr. What is the vapor pressure of a solution made from dissolving 11.5 g of biphenyl in 31.9 g of benzene?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Biphenyl, C12H10, is a nonvolatile, nonionizing solute that is soluble in benzene, C6H6. At 25 ∘C, t...
Questions

English, 08.01.2021 20:10


Mathematics, 08.01.2021 20:10






Mathematics, 08.01.2021 20:10

History, 08.01.2021 20:10

Mathematics, 08.01.2021 20:10

Chemistry, 08.01.2021 20:10


Mathematics, 08.01.2021 20:10

Mathematics, 08.01.2021 20:10




Physics, 08.01.2021 20:20

Mathematics, 08.01.2021 20:20


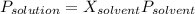 (1)
(1)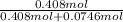 = 0.846
= 0.846