
Chemistry, 09.03.2020 18:37 alannadiaz1
Consider the reversible reaction A ( g ) − ⇀ ↽ − B ( g ) Which K values would indicate that there is more B than A at equilibrium? K = 9 × 10 – 5 K = 0.4 K = 5000 K = 8 × 10 7

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
Consider the reversible reaction A ( g ) − ⇀ ↽ − B ( g ) Which K values would indicate that there is...
Questions

History, 24.06.2019 12:40


Mathematics, 24.06.2019 12:40



Mathematics, 24.06.2019 12:40

Chemistry, 24.06.2019 12:40


Mathematics, 24.06.2019 12:40

Mathematics, 24.06.2019 12:40

Physics, 24.06.2019 12:40

English, 24.06.2019 12:40

English, 24.06.2019 12:40



History, 24.06.2019 12:40

Arts, 24.06.2019 12:40



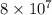 indicate that there is more B than A at equilibrium
indicate that there is more B than A at equilibrium![K=\frac{[B]}{[A]}](/tpl/images/0538/9301/3ac29.png)
![K=\frac{[B]}{[A]}1](/tpl/images/0538/9301/08f8d.png)


