
Chemistry, 09.03.2020 16:59 BigDough9090
375 mL of a 0.88 M potassium hydroxide solution is added to 496 mL of a 0.76 M cesium hydroxide solution. Calculate the pOH of the resulting solution.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
375 mL of a 0.88 M potassium hydroxide solution is added to 496 mL of a 0.76 M cesium hydroxide solu...
Questions

Mathematics, 14.04.2020 19:44


History, 14.04.2020 19:44


Geography, 14.04.2020 19:44



Mathematics, 14.04.2020 19:44


English, 14.04.2020 19:44


Mathematics, 14.04.2020 19:44




Geography, 14.04.2020 19:44

Mathematics, 14.04.2020 19:44



History, 14.04.2020 19:44

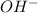 in 375 mL of 0.88 M of KOH =
in 375 mL of 0.88 M of KOH = 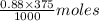 = 0.33 moles
= 0.33 moles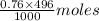 = 0.38 moles
= 0.38 moles![[OH^{-}]](/tpl/images/0538/8908/e46dd.png) =
= 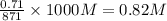
![pOH=-log[OH^{-}]=-log(0.82)=0.086](/tpl/images/0538/8908/1f1db.png)


