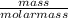What causes gas particles to collide with the walls of their containers?

Chemistry, 09.03.2020 06:46 stevencardona12mejia
Question 1.
What causes gas particles to collide with the walls of their containers?
A : Their volume
B : Their constant motion
C : Their pressure
D : Their small size
Question 2.
A balloon is filled with 5 grams of Helium gas. If the balloon takes up 10 mL of space and has a pressure of 20 mm Hg, which gas law needs to be used to find the temperature inside the balloon?
A: Charles law
B: Boyle’s law
C: Ideal gas law
D: Combined gas law

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Question 1.
What causes gas particles to collide with the walls of their containers?
What causes gas particles to collide with the walls of their containers?
Questions


Biology, 01.08.2019 03:30


Mathematics, 01.08.2019 03:30

History, 01.08.2019 03:30




History, 01.08.2019 03:30



Social Studies, 01.08.2019 03:30



Biology, 01.08.2019 03:30

Physics, 01.08.2019 03:30

Biology, 01.08.2019 03:30

Biology, 01.08.2019 03:30

Chemistry, 01.08.2019 03:30