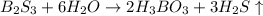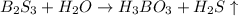Chemistry, 08.03.2020 07:01 ashlee7877
Boron sulfide, B2S3(s), reacts violently with water to form dissolved boric acid (H3BO3) and hydrogen sulfide gas

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
You know the right answer?
Boron sulfide, B2S3(s), reacts violently with water to form dissolved boric acid (H3BO3) and hydroge...
Questions


Mathematics, 17.02.2021 23:30


Mathematics, 17.02.2021 23:30



Biology, 17.02.2021 23:30

English, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30


Mathematics, 17.02.2021 23:30






History, 17.02.2021 23:30