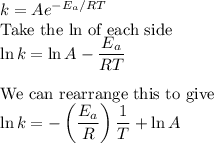Chemistry, 07.03.2020 06:12 janellesteele7498
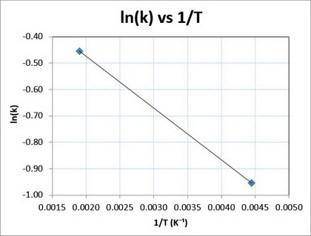
Consider the reaction data.
A⟶products
T () (−1)
225 0.394
675 0.812
What two points should be plotted to graphically determine the activation energy of this reaction? To avoid rounding errors, use at least three significant figures in all values.
x1=
y1=
x2=
y2=
Determine the rise, run, and slope of the line formed by these points.
rise:
run:
slope:
What is the activation energy of this reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
Consider the reaction data.
A⟶products
T () (−1)
225 0.394
675...
A⟶products
T () (−1)
225 0.394
675...
Questions

Mathematics, 02.04.2021 02:10




Health, 02.04.2021 02:10






Chemistry, 02.04.2021 02:20


Health, 02.04.2021 02:20


Social Studies, 02.04.2021 02:20





Mathematics, 02.04.2021 02:20