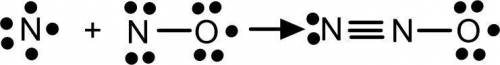Chemistry, 07.03.2020 05:58 catsareokiguess
Many free radicals combine to form molecules that do not contain any unpaired electrons. The driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. Consider the chemical equation. N ( g ) + NO ( g ) ⟶ NNO ( g ) Write Lewis formulas for the reactant and product species in the chemical equation. Include nonbonding electrons.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
You know the right answer?
Many free radicals combine to form molecules that do not contain any unpaired electrons. The driving...
Questions



Mathematics, 26.06.2019 01:30





Social Studies, 26.06.2019 01:30

History, 26.06.2019 01:30



Chemistry, 26.06.2019 01:30

Mathematics, 26.06.2019 01:30

Mathematics, 26.06.2019 01:30

Chemistry, 26.06.2019 01:30


Biology, 26.06.2019 01:30


Mathematics, 26.06.2019 01:30