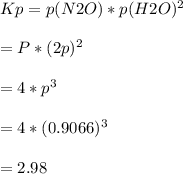Chemistry, 07.03.2020 06:17 missheather0309
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed explosively according to the following equation:
NH4NO3(s) N2O(g) + 2H2O(g)
At equilibrium, the total pressure in the container was found to be 2.72 bar at a temperature of 500.°C. Calculate Kp.
a.
1.64
b.
0.822
c.
2.98
d.
80.5
e.
0.745

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 12:30
What are some examples of anthropogenic atmospheric particulates?
Answers: 1


Chemistry, 23.06.2019 18:00
Based on the data in the map where would you expect vegitation on earth to be more dense
Answers: 2

Chemistry, 23.06.2019 21:00
Using the periodic table choose the more reactive nonmetal s or as
Answers: 1
You know the right answer?
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed...
Questions


Mathematics, 08.09.2021 21:10


English, 08.09.2021 21:10







Chemistry, 08.09.2021 21:10


History, 08.09.2021 21:10

Biology, 08.09.2021 21:10



Mathematics, 08.09.2021 21:10

Business, 08.09.2021 21:10

Mathematics, 08.09.2021 21:10

Mathematics, 08.09.2021 21:10