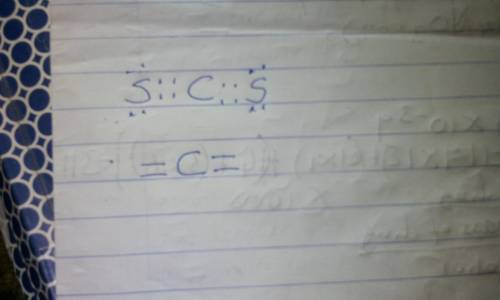Chemistry, 07.03.2020 05:27 kandrews6221
Draw the Lewis structure for each of the following and THEN determine the electron-pair geometry of the atom indicated. Do not include formal charges in your drawing. C in CS2 : electron-pair geometry

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
Draw the Lewis structure for each of the following and THEN determine the electron-pair geometry of...
Questions

English, 25.07.2021 14:00

Mathematics, 25.07.2021 14:00

Mathematics, 25.07.2021 14:00

Mathematics, 25.07.2021 14:00

Mathematics, 25.07.2021 14:00

Mathematics, 25.07.2021 14:00



English, 25.07.2021 14:00

Mathematics, 25.07.2021 14:00

Mathematics, 25.07.2021 14:00

English, 25.07.2021 14:00

Health, 25.07.2021 14:00

Mathematics, 25.07.2021 14:00

Mathematics, 25.07.2021 14:00

Mathematics, 25.07.2021 14:00



Mathematics, 25.07.2021 14:00

English, 25.07.2021 14:00