Chemistry, 07.03.2020 05:26 kfcnkfnmnfk9513
A sample of pure NO2 is heated to 337?C at which temperature it partially dissociates according to the equation2NO2(g)?2NO(g)+O2(g)At equilibrium the density of the gas mixture is 0.525g/L at 0.745atm . Calculate Kc for the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
You know the right answer?
A sample of pure NO2 is heated to 337?C at which temperature it partially dissociates according to t...
Questions



Social Studies, 05.11.2019 08:31



Computers and Technology, 05.11.2019 08:31






Mathematics, 05.11.2019 08:31

World Languages, 05.11.2019 08:31



Chemistry, 05.11.2019 08:31


English, 05.11.2019 08:31

Physics, 05.11.2019 08:31

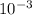
![\frac{[NO]^{2} .[O2]}{[NO2]^{2} }](/tpl/images/0537/9415/78cff.png)
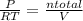
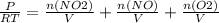
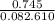 = 0.015 mol/L
= 0.015 mol/L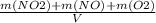
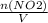 +M(NO)·
+M(NO)·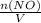 +M(O2)·
+M(O2)·