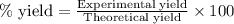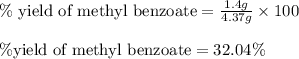Chemistry, 07.03.2020 05:25 livvyr0cks
Consider the Fischer ester synthesis of methyl benzoate from benzoic acid and methanol in the presence of sulfuric acid as a catalyst. A reaction was performed in which 3.6 g of benzoic acid was reacted with excess methanol to make 1.4 g of methyl benzoate. Calculate the theoretical yield and percent yield for this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
You know the right answer?
Consider the Fischer ester synthesis of methyl benzoate from benzoic acid and methanol in the presen...
Questions








Computers and Technology, 10.03.2020 18:47

Mathematics, 10.03.2020 18:47





Chemistry, 10.03.2020 18:48

Mathematics, 10.03.2020 18:48




Mathematics, 10.03.2020 18:48

Computers and Technology, 10.03.2020 18:48

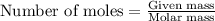 .....(1)
.....(1)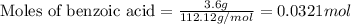

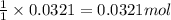 of methyl benzoate
of methyl benzoate