
Chemistry, 07.03.2020 05:15 ReeseMoffitt8032
The Lewis electron-dot diagrams of the HClO3 molecule and the HClO2molecule are shown above at the left and right, respectively.
Which of the following statements identifies the stronger acid and correctly identifies a factor that contributes to its being the stronger acid?
(A)HClO3(aq)is the stronger acid because its molecules experience stronger Londondispersion forces.
(B)HClO3(aq)is the stronger acid because the additional electronegative oxygen atom on the chlorine atom stabilizes the conjugate base.
(C)HClO2(aq)molecules experience weaker London dispersion forces. is the stronger acid because its
(D)HClO2(aq)is the stronger acid because the Acid Solution Volume of NaOH Added (mL) A40B75C115D200lone pairs of electrons on the chlorine atom stabilize the conjugate base.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

You know the right answer?
The Lewis electron-dot diagrams of the HClO3 molecule and the HClO2molecule are shown above at the l...
Questions

Mathematics, 04.06.2021 19:50


English, 04.06.2021 19:50


Mathematics, 04.06.2021 19:50

Arts, 04.06.2021 19:50


Social Studies, 04.06.2021 19:50


Chemistry, 04.06.2021 19:50

Mathematics, 04.06.2021 19:50

French, 04.06.2021 19:50

Arts, 04.06.2021 19:50


Mathematics, 04.06.2021 19:50


Mathematics, 04.06.2021 19:50

English, 04.06.2021 19:50

Mathematics, 04.06.2021 19:50

History, 04.06.2021 19:50

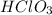 has three electronegative oxygen atoms and
has three electronegative oxygen atoms and 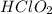 has two electronegative oxygen atoms
has two electronegative oxygen atoms

