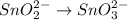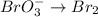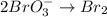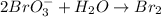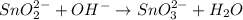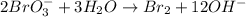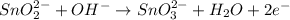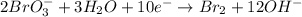Chemistry, 07.03.2020 04:59 rileyeddins1010
2BrO3- + 5SnO22-+ H2O5SnO32- + Br2+ 2OH- In the above reaction, the oxidation state of tin changes from to . How many electrons are transferred in the reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
2BrO3- + 5SnO22-+ H2O5SnO32- + Br2+ 2OH- In the above reaction, the oxidation state of tin changes f...
Questions

Mathematics, 05.08.2020 02:01

Biology, 05.08.2020 02:01


Mathematics, 05.08.2020 02:01

Biology, 05.08.2020 02:01





Mathematics, 05.08.2020 02:01

History, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01

History, 05.08.2020 02:01



Mathematics, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01

Mathematics, 05.08.2020 02:01


Mathematics, 05.08.2020 02:01

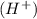 at that side where the less number of hydrogen are present.
at that side where the less number of hydrogen are present.