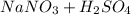Chemistry, 07.03.2020 04:38 isiahemerson0
Write a net ionic equation for the reaction that occurs when excess nitric acid (aq) and sodium sulfite (aq) are combined. Note: Sulfites follow the same solubility trends as sulfates.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
Write a net ionic equation for the reaction that occurs when excess nitric acid (aq) and sodium sulf...
Questions

Mathematics, 14.01.2021 01:00


Mathematics, 14.01.2021 01:00



Mathematics, 14.01.2021 01:00


Business, 14.01.2021 01:00


Mathematics, 14.01.2021 01:00


Geography, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

Arts, 14.01.2021 01:00




SAT, 14.01.2021 01:00

Mathematics, 14.01.2021 01:00

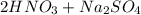 ⇒
⇒ 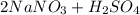
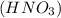
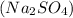
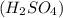
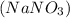
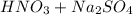 ⇒
⇒