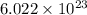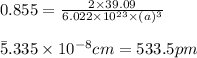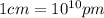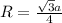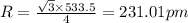Chemistry, 07.03.2020 04:28 winterblanco
The density of potassium, which has the BCC structure, is 0.855 g/cm3. The atomic weight of potassium is 39.09 g/mol. Calculate (a) the lattice parameter; and (b) the atomic radius of potassium.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2
You know the right answer?
The density of potassium, which has the BCC structure, is 0.855 g/cm3. The atomic weight of potassiu...
Questions


Arts, 26.01.2021 18:20

Chemistry, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20

English, 26.01.2021 18:20




Mathematics, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20

English, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20


Mathematics, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20

World Languages, 26.01.2021 18:20

Mathematics, 26.01.2021 18:20


Mathematics, 26.01.2021 18:20

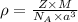
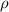 = density =
= density = 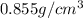
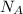 = Avogadro's number =
= Avogadro's number =