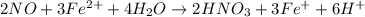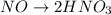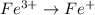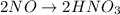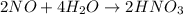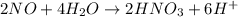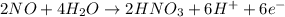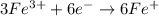Chemistry, 07.03.2020 04:45 phillipsalexis274
2NO + 3Fe2++ 4H2O2HNO3 + 3Fe+ 6H+ In the above reaction, the oxidation state of nitrogen changes from to . How many electrons are transferred in the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
2NO + 3Fe2++ 4H2O2HNO3 + 3Fe+ 6H+ In the above reaction, the oxidation state of nitrogen changes fro...
Questions

Chemistry, 03.10.2020 01:01


Health, 03.10.2020 01:01

Health, 03.10.2020 01:01

Mathematics, 03.10.2020 01:01


Mathematics, 03.10.2020 01:01



English, 03.10.2020 01:01

Social Studies, 03.10.2020 01:01

Engineering, 03.10.2020 01:01


Mathematics, 03.10.2020 01:01


Mathematics, 03.10.2020 01:01

Chemistry, 03.10.2020 01:01

Biology, 03.10.2020 01:01


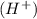 at that side where the less number of hydrogen are present.
at that side where the less number of hydrogen are present.