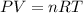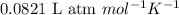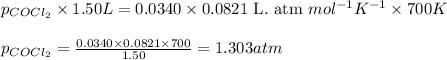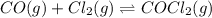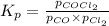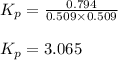Chemistry, 07.03.2020 04:27 fredorivera
Pure phosgene gas (COCl2), 0.0340 mol, was placed in a 1.50−L container. It was heated to 700.0 K, and at equilibrium, the pressure of CO was found to be 0.509 atm. Calculate the equilibrium constant KP for the reaction. CO(g) + Cl2(g) ⇌ COCl2(g)KP =

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
Pure phosgene gas (COCl2), 0.0340 mol, was placed in a 1.50−L container. It was heated to 700.0 K, a...
Questions


History, 19.05.2021 20:00



English, 19.05.2021 20:00

Social Studies, 19.05.2021 20:00

Mathematics, 19.05.2021 20:00

History, 19.05.2021 20:00


English, 19.05.2021 20:00


Mathematics, 19.05.2021 20:00



English, 19.05.2021 20:00



History, 19.05.2021 20:00


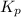 for the given equation is 3.065
for the given equation is 3.065