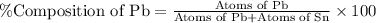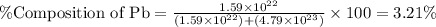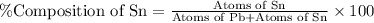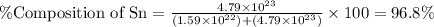Chemistry, 07.03.2020 04:57 fatherbamboo
What is the composition, in atom percent, of an alloy that consists of a) 5.5 wt% Pb and b) 94.5 wt% of Sn? Assume that the atomic weight for lead and tin are 207.2 and 118.71 g/mol, respectively.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

You know the right answer?
What is the composition, in atom percent, of an alloy that consists of a) 5.5 wt% Pb and b) 94.5 wt%...
Questions

Mathematics, 19.11.2020 19:50

Health, 19.11.2020 19:50


Computers and Technology, 19.11.2020 19:50


Arts, 19.11.2020 19:50


Mathematics, 19.11.2020 19:50

Mathematics, 19.11.2020 19:50


Mathematics, 19.11.2020 19:50

Mathematics, 19.11.2020 19:50



Mathematics, 19.11.2020 19:50

Biology, 19.11.2020 19:50


Mathematics, 19.11.2020 19:50

Mathematics, 19.11.2020 19:50

Health, 19.11.2020 19:50

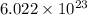 atoms
atoms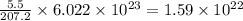 atoms
atoms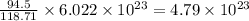 atoms
atoms