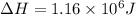Chemistry, 07.03.2020 05:14 spdesch2558
Calculate the amount of energy (in kilojoules) needed to heat 346 g of liquid water from –10 °C to 182°C. Assume that the specific heat of water is 4.184 J/g · °C for liquid and that the specific heat of steam is 1.99 J/g · °C.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Calculate the amount of energy (in kilojoules) needed to heat 346 g of liquid water from –10 °C to 1...
Questions







Mathematics, 21.09.2021 09:30







Mathematics, 21.09.2021 09:30

Physics, 21.09.2021 09:30


Mathematics, 21.09.2021 09:30

Biology, 21.09.2021 09:30

Business, 21.09.2021 09:30

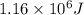
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+m\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+m\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0537/8966/4a4bb.png)
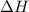 = heat required for the reaction
= heat required for the reaction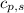 = specific heat of solid water or ice =
= specific heat of solid water or ice = 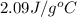
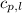 = specific heat of liquid water =
= specific heat of liquid water = 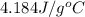
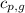 = specific heat of gaseous water =
= specific heat of gaseous water = 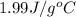
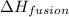 = enthalpy change for fusion =
= enthalpy change for fusion = 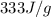
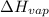 = enthalpy change for vaporization =
= enthalpy change for vaporization = 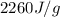
![\Delta H=[346g\times 2.09J/g^oC\times (0-(-80))^oC]+346g\times 333J/g+[346g\times 4.184J/g^oC\times (100-0)^oC]+346g\times 2260J/g+[346g\times 1.99J/g^oC\times (180-100)^oC]](/tpl/images/0537/8966/7d24b.png)