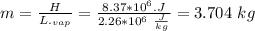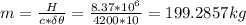Chemistry, 07.03.2020 03:56 damiangibson2
Assume that a daily diet of 2000 calories (i. e. 8.37 x 106 J) is converted completely to body heat.
a) How many g of water (as sweat) would need to evaporate to cool that person off?
b) If instead of evaporating water, the heat was used to raise the temperature of some water from 25.0 °C to 35.0 °C, how much water could be heated?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1
You know the right answer?
Assume that a daily diet of 2000 calories (i. e. 8.37 x 106 J) is converted completely to body heat....
Questions

Mathematics, 19.01.2021 19:00

Mathematics, 19.01.2021 19:00



Mathematics, 19.01.2021 19:00


History, 19.01.2021 19:00




Biology, 19.01.2021 19:00





Mathematics, 19.01.2021 19:00


Advanced Placement (AP), 19.01.2021 19:00

Mathematics, 19.01.2021 19:00