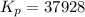Chemistry, 07.03.2020 03:29 tashakelly42
Consider the following reaction N2(g) + 3H2(g) 2NH3(g)0.866 atm of N2 and 0.0496 atm of H2 are placed in a flask; when equilibrium is reached, the pressure of NH3 is 0.0310 atm. Calculate Kpfor the reaction. Use the following steps to solvethis problem:

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Consider the following reaction N2(g) + 3H2(g) 2NH3(g)0.866 atm of N2 and 0.0496 atm of H2 are place...
Questions


History, 19.11.2020 01:50

Mathematics, 19.11.2020 01:50


Mathematics, 19.11.2020 01:50

Arts, 19.11.2020 01:50



Mathematics, 19.11.2020 01:50

History, 19.11.2020 01:50


Mathematics, 19.11.2020 01:50





Advanced Placement (AP), 19.11.2020 01:50



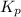 is 37928.
is 37928.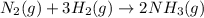
![K_p=\frac{[p_{NH_3}]^2}{p_{N_2}\times [p_{H_2}]^3}](/tpl/images/0537/3920/ee3ee.png)
![K_p=\frac{[2x]^2}{(0.866-x)\times (0.0496-3x)^3}](/tpl/images/0537/3920/b2858.png)