This is an incomplete question, here is a complete question.
Classify the following reaction: 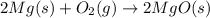
a. oxidation-reduction
b. combustion
c. synthesis
d. two of the above
e. a-c are all correct.
Answer : The correct option is, (e) a-c are all correct.
Explanation :
Synthesis reaction : A chemical reaction where multiple substances or reactants combine to form a single product.
It is represented as,
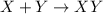
Combustion reaction : A chemical reaction in which a hydrocarbon reaction with the oxygen to give product as carbon dioxide and water.
It is represented as,
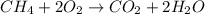
Oxidation-reduction reaction : It is defined as the reaction in which the oxidation and reduction reaction takes place simultaneously.
Oxidation reaction : It is defined as the reaction in which a substance looses its electrons. In this, oxidation state of an element increases.
Reduction reaction : It is defined as the reaction in which a substance gains electrons. In this, oxidation state of an element decreases.
The given chemical reaction is:
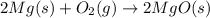
It is an oxidation-reduction reaction in which Mg shows oxidation because the oxidation state of Mg changes from (0) to (+2) and O shows reduction because the oxidation state of O changes from (0) to (-2).
This reaction is also a synthesis reaction because two reactants combine to form single product.
Hence, the correct option is, (e) a-c are all correct.



























