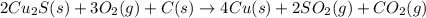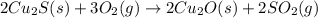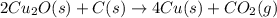There are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first step, copper(I) sulfide and oxygen react to form copper(I) oxide and sulfur dioxide: (s)(g)(s)(g) In the second step, copper(I) oxide and carbon react to form copper and carbon monoxide: (s)(s)(s)(g) Write the net chemical equation for the production of copper from copper(I) sulfide, oxygen and carbon. Be sure your equation is balanced.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
There are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first st...
Questions




Physics, 20.08.2019 21:30


Mathematics, 20.08.2019 21:30

English, 20.08.2019 21:30

Geography, 20.08.2019 21:30



Advanced Placement (AP), 20.08.2019 21:30

Mathematics, 20.08.2019 21:30

Biology, 20.08.2019 21:30

History, 20.08.2019 21:30

Mathematics, 20.08.2019 21:30


Biology, 20.08.2019 21:30

Mathematics, 20.08.2019 21:30


Mathematics, 20.08.2019 21:30