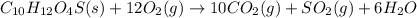Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3

You know the right answer?
C10H12O4S(s) + O2(g) CO2(g) + SO2(g) + H2O(g)
26. When the equation above is balanced...
26. When the equation above is balanced...
Questions


Social Studies, 13.07.2019 03:50



Biology, 13.07.2019 03:50

History, 13.07.2019 03:50

Mathematics, 13.07.2019 03:50


Business, 13.07.2019 03:50

Business, 13.07.2019 03:50



Biology, 13.07.2019 03:50


Social Studies, 13.07.2019 03:50

History, 13.07.2019 03:50

English, 13.07.2019 03:50

English, 13.07.2019 03:50

Mathematics, 13.07.2019 03:50

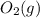 is 12.
is 12.