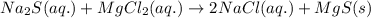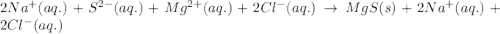Chemistry, 07.03.2020 03:12 foodisbae45678
An aqueous solution of sodium sulfide is allowed to react with an aqueous solution of magnesium chloride. The complete ionic equation contains which of the following species (when balanced in standard form)?a. Cl-(aq)b.2Na+(aq)c.2S2-(aq)d. Na+(aq)e.3Mg2+(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
An aqueous solution of sodium sulfide is allowed to react with an aqueous solution of magnesium chlo...
Questions





Mathematics, 17.01.2020 05:31



Computers and Technology, 17.01.2020 05:31






Computers and Technology, 17.01.2020 05:31






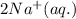 ions
ions