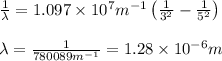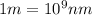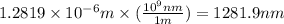Chemistry, 07.03.2020 02:47 mailani12503
Recall Planck's constant equals 6.63 × 10−34 J·s and the speed of light is 3.00 × 108 m/s. Calculate the wavelength (in nm) of a photon emitted by a hydrogen atom when its electron drops from the n = 5 state to the n = 3 state.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 13:30
The activation energy for a(n) is quite large and usually takes extra energy from the environment, it is normally not a natural spontaneous process. combustion reaction endothermic reaction exothermic reaction catalyzed reaction
Answers: 3

Chemistry, 23.06.2019 18:30
Esure to answer all parts. the equilibrium constant for the reaction ni2+(aq) + 6 nh3(aq) ⇌ ni(nh3)6 2+(aq) is kf = 5.6 × 108 at 25°c. (a) what is δg o at this temperature? (b) if standard-state concentrations of reactants and products are mixed, in which direction does the reaction proceed? (c) determine δg when [ni(nh3)62+] = 0.010 m, [ni2+] = 0.0010 m, and [nh3] = 0.0050 m. in which direction will the reaction proceed to achieve equilibrium? (a) × 10 j/mol (enter your answer in scientific notation.) (b) to the right. to the left. (c) × 10 j/mol (enter your answer in scientific notation.) to the right. to the left.
Answers: 3
You know the right answer?
Recall Planck's constant equals 6.63 × 10−34 J·s and the speed of light is 3.00 × 108 m/s. Calculate...
Questions


Mathematics, 16.10.2020 15:01

English, 16.10.2020 15:01

History, 16.10.2020 15:01

History, 16.10.2020 15:01





Biology, 16.10.2020 15:01


Biology, 16.10.2020 15:01


Arts, 16.10.2020 15:01



Mathematics, 16.10.2020 15:01


Arts, 16.10.2020 15:01

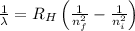
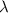 = Wavelength of radiation
= Wavelength of radiation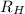 = Rydberg's Constant =
= Rydberg's Constant = 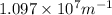
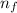 = Upper energy level = 3
= Upper energy level = 3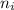 = Lower energy level = 5
= Lower energy level = 5