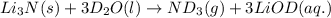Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation Li 3 N ( s ) + 3 H 2 O ( l ) ⟶ NH 3 ( g ) + 3 LiOH ( aq ) Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D 2 O . The same reaction can be used to produce heavy ammonia, ND 3 ( g ) , according to the equation Li 3 N ( s ) + 3 D 2 O ( l ) ⟶ ND 3 ( g ) + 3 LiOD ( aq ) Calculate how many grams of heavy water are required to produce 455.0 mg ND 3 ( g ) . The mass of deuterium, D , is 2.014 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation...
Questions

Mathematics, 02.01.2021 21:20

Mathematics, 02.01.2021 21:20




Mathematics, 02.01.2021 21:20

History, 02.01.2021 21:20

Mathematics, 02.01.2021 21:20



Biology, 02.01.2021 21:20



Mathematics, 02.01.2021 21:20


Mathematics, 02.01.2021 21:30

Mathematics, 02.01.2021 21:30


Mathematics, 02.01.2021 21:30

 needed is 1.36 grams
needed is 1.36 grams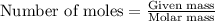 .....(1)
.....(1)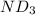 :
: