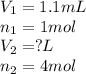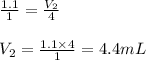Chemistry, 07.03.2020 02:29 homework1911
Methane gas and chlorine gas react to form hydrogen chloride gas and carbon tetrachloride gas. What volume of hydrogen chloride would be produced by this reaction if of methane were consumed? Also, be sure your answer has a unit symbol, and

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Methane gas and chlorine gas react to form hydrogen chloride gas and carbon tetrachloride gas. What...
Questions


Health, 25.11.2020 03:50

Mathematics, 25.11.2020 03:50

English, 25.11.2020 03:50

English, 25.11.2020 03:50

Mathematics, 25.11.2020 03:50

Physics, 25.11.2020 03:50






English, 25.11.2020 03:50





Physics, 25.11.2020 03:50


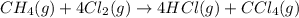
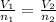
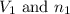 are the volume and number of moles of methane gas
are the volume and number of moles of methane gas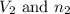 are the volume and number of moles of hydrogen chloride
are the volume and number of moles of hydrogen chloride