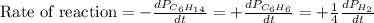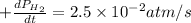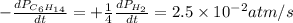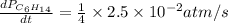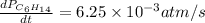Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1
You know the right answer?
For the reaction C6H14(g) > C6H6(g) + 4H2(g), the rate of formation of hydrogen gas, H2 was found...
Questions

Chemistry, 11.07.2019 15:30

Chemistry, 11.07.2019 15:30







Social Studies, 11.07.2019 15:30

Social Studies, 11.07.2019 15:30



Biology, 11.07.2019 15:30



English, 11.07.2019 15:30


Mathematics, 11.07.2019 15:30


Chemistry, 11.07.2019 15:30

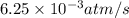
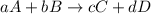
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0537/1900/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0537/1900/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0537/1900/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0537/1900/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0537/1900/d4b94.png)
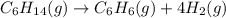
![\text{Rate of disappearance of }C_6H_{14}=-\frac{d[C_6H_{14}]}{dt}](/tpl/images/0537/1900/89957.png)
![\text{Rate of formation of }C_6H_6=+\frac{d[C_6H_6]}{dt}](/tpl/images/0537/1900/d8137.png)
![\text{Rate of formation of }H_2=+\frac{1}{4}\frac{d[H_2]}{dt}](/tpl/images/0537/1900/ecdf8.png)