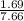Chemistry, 07.03.2020 02:47 garretthyatt123
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 200. mL flask with 2.6 atm of sulfur dioxide gas and 0.90 atm of oxygen gas at 35.°C. She then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 1.3 atm. Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions

Social Studies, 17.07.2019 10:40

Mathematics, 17.07.2019 10:40




Social Studies, 17.07.2019 10:40




History, 17.07.2019 10:40

English, 17.07.2019 10:40

Computers and Technology, 17.07.2019 10:50


Biology, 17.07.2019 10:50


Social Studies, 17.07.2019 10:50


Mathematics, 17.07.2019 10:50


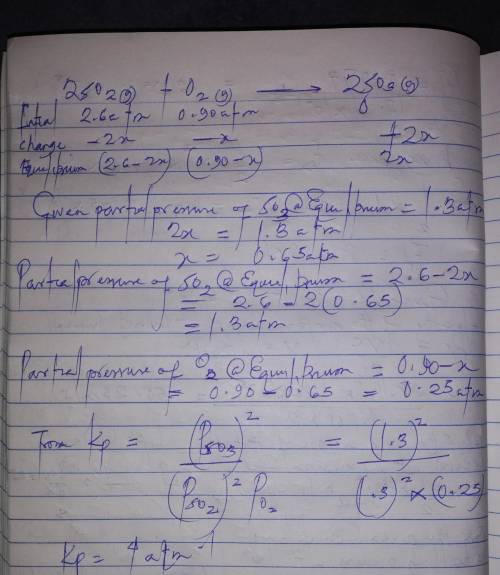
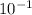 = 0.22 to 2 sig. fig.
= 0.22 to 2 sig. fig.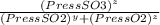
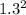 ÷ (
÷ (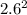 + 0.9)
+ 0.9)