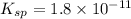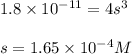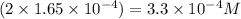Chemistry, 07.03.2020 02:24 avisconti571
A 350 mL saturated solution of magnesium hydroxide, Mg(OH)2 is prepared at 25 degrees Celsius. The solubility product constant, Ksp, for Mg(OH)2 is 1.8 x 10^-11 at 25 degrees Celsius.
1) Find the molar solubility of Mg(OH)2 at 25 degrees Celsius?
2) What is the concentration (mol/L) of hydroxide ion, OH-, in the saturated solution at 25 degrees Celsius?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
A 350 mL saturated solution of magnesium hydroxide, Mg(OH)2 is prepared at 25 degrees Celsius. The s...
Questions

Mathematics, 03.04.2020 00:24




History, 03.04.2020 00:24





English, 03.04.2020 00:25

Geography, 03.04.2020 00:25


Mathematics, 03.04.2020 00:25


English, 03.04.2020 00:25

Biology, 03.04.2020 00:25


History, 03.04.2020 00:25


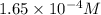
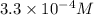
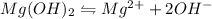
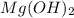 will be:
will be:![K_{sp}=[Mg^{2+}][OH^-]^2\\\\K_{sp}=s\times (2s)^2=4s^3](/tpl/images/0537/0995/7d1c0.png)