Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
What minimum mass of iron (II) nitrate must be added to 10.0 of a 0.0699 M phosphate solution in ord...
Questions

English, 17.09.2019 04:00

Geography, 17.09.2019 04:00

Mathematics, 17.09.2019 04:00

Computers and Technology, 17.09.2019 04:00

Mathematics, 17.09.2019 04:00

Mathematics, 17.09.2019 04:00


Mathematics, 17.09.2019 04:00









Mathematics, 17.09.2019 04:00


Biology, 17.09.2019 04:00

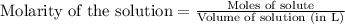

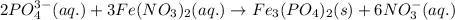
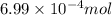 of phosphate solution will react with =
of phosphate solution will react with = 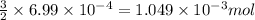 of iron (II) nitrate
of iron (II) nitrate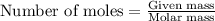
 moles
moles