Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

You know the right answer?
The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an acti...
Questions

Mathematics, 27.01.2021 22:00

History, 27.01.2021 22:00


History, 27.01.2021 22:00



Mathematics, 27.01.2021 22:00

Mathematics, 27.01.2021 22:00





Mathematics, 27.01.2021 22:00

Mathematics, 27.01.2021 22:00


History, 27.01.2021 22:00


Mathematics, 27.01.2021 22:00


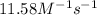
![\ln(\frac{K_{56.0^oC}}{K_{-8.0^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0536/9043/5e447.png)
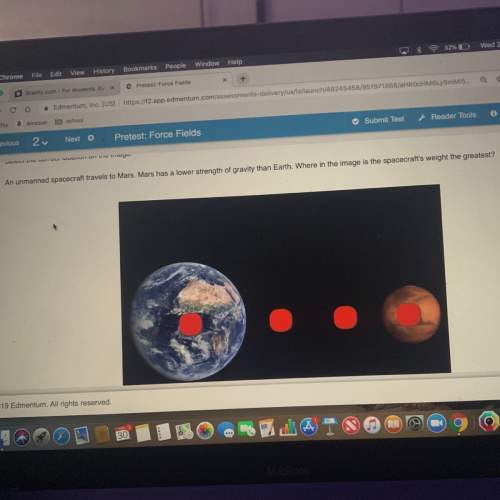
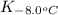 = equilibrium constant at -8.0°C =
= equilibrium constant at -8.0°C = 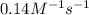
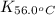 = equilibrium constant at 56°C = ?
= equilibrium constant at 56°C = ?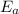 = Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)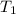 = initial temperature =
= initial temperature = ![-8.0^oC=[273-8]K=265K](/tpl/images/0536/9043/f4197.png)
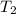 = final temperature =
= final temperature = ![56.0^oC=[273+56]K=329K](/tpl/images/0536/9043/23b22.png)
![\ln(\frac{K_{56.0^oC}}{0.14})=\frac{50000J}{8.314J/mol.K}[\frac{1}{265}-\frac{1}{329}]\\\\K_{56.0^oC}=11.58M^{-1}s^{-1}](/tpl/images/0536/9043/35497.png)