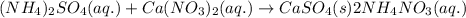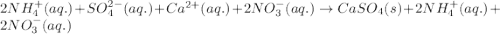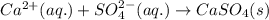Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

You know the right answer?
An aqueous solution of ammonium sulfate is allowed to react with an aqueous solution of calcium nitr...
Questions




History, 01.10.2019 22:40


History, 01.10.2019 22:40

Mathematics, 01.10.2019 22:40

Mathematics, 01.10.2019 22:40



Mathematics, 01.10.2019 22:40

Computers and Technology, 01.10.2019 22:40

History, 01.10.2019 22:40

Mathematics, 01.10.2019 22:40

Social Studies, 01.10.2019 22:40





Physics, 01.10.2019 22:40

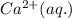 ions
ions