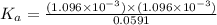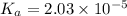Chemistry, 07.03.2020 00:05 jessicaortiz6
Using the average initial pH of your acetic acid solutions (2.96), and the average molarity of those solutions(.0602), calculate a value of Ka for acetic acid. Report your result to 3 significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

You know the right answer?
Using the average initial pH of your acetic acid solutions (2.96), and the average molarity of those...
Questions

English, 29.09.2019 07:10


Mathematics, 29.09.2019 07:10

Mathematics, 29.09.2019 07:10




Arts, 29.09.2019 07:10

History, 29.09.2019 07:10


Spanish, 29.09.2019 07:10


Mathematics, 29.09.2019 07:10

Mathematics, 29.09.2019 07:10


Geography, 29.09.2019 07:10

Mathematics, 29.09.2019 07:10


Mathematics, 29.09.2019 07:10

Physics, 29.09.2019 07:10

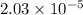
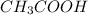 .
.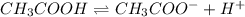
![pH=-\log [H^+]](/tpl/images/0536/6580/37e81.png)
![2.96=-\log [H^+]](/tpl/images/0536/6580/04be1.png)
![[H^+]=1.096\times 10^{-3}M](/tpl/images/0536/6580/640ad.png)
![[H^+]=[CH_3COO^-]=1.096\times 10^{-3}M](/tpl/images/0536/6580/4b8c0.png)
![[CH_3COOH]=0.0602-(1.096\times 10^{-3})=0.0591M](/tpl/images/0536/6580/19210.png)
![K_a=\frac{[CH_3COO^-][H^+]}{[CH_3COOH]}](/tpl/images/0536/6580/9c35d.png)