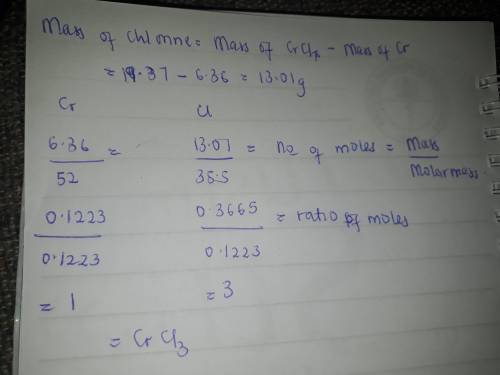Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
When 6.36 g of metallic chromium is heated with elemental chlorine gas, 19.37 g of a chromium chlori...
Questions


Mathematics, 01.03.2021 22:20

Social Studies, 01.03.2021 22:20


Social Studies, 01.03.2021 22:20


Chemistry, 01.03.2021 22:20

Advanced Placement (AP), 01.03.2021 22:20


Biology, 01.03.2021 22:20






Arts, 01.03.2021 22:20



Mathematics, 01.03.2021 22:20