
Chemistry, 07.03.2020 00:25 luisgonz5050
If 0.220 mol of solid CaCO3 and 670 mL of 0.433 M aqueous H2SO4 are reacted stoichiometrically according to the balanced equation. How many grams of solid CaSO4 are produced?
H2SO4(aq) + CaCO3(s) → CO2(g) + CaSO4(s) + H2O(l)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
If 0.220 mol of solid CaCO3 and 670 mL of 0.433 M aqueous H2SO4 are reacted stoichiometrically accor...
Questions

Mathematics, 12.11.2019 02:31








Computers and Technology, 12.11.2019 02:31











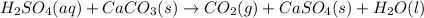
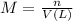
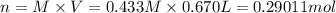
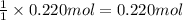 sulfuric acid.
sulfuric acid.

