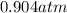Chemistry, 06.03.2020 23:49 RoyalGurl01
A sealed container holding 0.0255 L of an ideal gas at 0.979 atm and 67 ∘ C is placed into a refrigerator and cooled to 41 ∘ C with no change in volume. Calculate the final pressure of the gas. P = atm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2


Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
A sealed container holding 0.0255 L of an ideal gas at 0.979 atm and 67 ∘ C is placed into a refrige...
Questions

Social Studies, 10.12.2020 01:20

Mathematics, 10.12.2020 01:20


History, 10.12.2020 01:20


Mathematics, 10.12.2020 01:20

Mathematics, 10.12.2020 01:20



Geography, 10.12.2020 01:20

Mathematics, 10.12.2020 01:20

Mathematics, 10.12.2020 01:20

Social Studies, 10.12.2020 01:20

Social Studies, 10.12.2020 01:20


Chemistry, 10.12.2020 01:20


English, 10.12.2020 01:20


English, 10.12.2020 01:20